Close Packed Structures and Packing Efficiency
Close Packed Structures and Packing Efficiency: Overview
This topic covers concepts, such as Coordination Number, Coordination Number of Simple Cubic Lattice, Coordination Number of BCC Lattice, Coordination Number of FCC or CCP Lattice, and Close Packing of Spheres in Crystalline Solids.
Important Questions on Close Packed Structures and Packing Efficiency
Silver crystallises with face-centred cubic unit cells and each side of the unit cell has a length of . Determine the radius of an atom of silver. (Assume that each face atom is touching the four corner atoms.)
A metallic element crystallises into lattice having a layering sequence of Any packing of sphere leaves out voids in the lattice. Determine what percentage by volume of this lattice is empty space.
The coordination number of a metal crystallizing in a hexagonal close-packed structure is:
has bcc structure with edge length . The shortest inter ionic distance in between and is:
The edge length of a face-centred cubic cell of an ionic substance is . If the radius of the cation is , the radius of anion is
What is the coordination number of sodium in sodium oxide ?
The number of octahedral sites per sphere in FCC structure is
In a compound, atoms of elements form ccp lattice and those of element occpy of tetrahedral voids. The formula of the compound will be
Percentage of free space in cubic close-packed structured and in a body centred packed structure are respectively
Interstitial sites that are formed when three closed packed spheres of one layer is put on three close packed spheres of the second layer, their positions being inverted with respect to each other.
A tetrahedral void in is formed by atoms at
Number of formula units in unit cell of (rock salt), (zinc blende) and (fcc) respectively
What is the coordination number of in unit cell if ionic radii of and ions being and respectively?
In normal spinel structure there is a closed packed array of ions. The trivalent cations are present in
has type of structure. The edge length of its unit cell has been found to be . The distance between in is
For tetrahedral co-ordination, the radius ratio should be
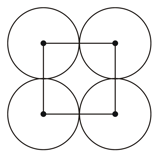
The packing efficiency of the square cell shown below is
In a BCC unit cell, if half of the atoms per unit cell are removed, then percentage void is
In a ccp type structure, if half of the face-centred atoms are removed, then percentage void in unit cell is approximately
The coordination number in one dimensional close packed arrangement is
